Welcome to the MDSO resources page
This page is reserved for members of the Medical Device Safety Officer (MDSO) network in the UK. Links to already established documents, reports and resources and content will be shared here. These resources are not considered to be confidential and can also be shared with professional colleagues working in medical device safety, risk and governance. Please check the ‘events’ section at the bottom of the main NAMDET website home page for upcoming meetings, MDSO Webex information and other conference dates and times. Please remember, the MDSO Webex events are booked for the first Wednesday of the month and run from 1 -2 p.m (GMT).
Follow these quick links to the main websites to keep up to date with any patient safety alerts, important safety information and share any content with your colleagues.

The current MDSO Editorial Board members can be viewed by downloading the document
MDSO: Editorial Board members 2022

MDSO Editorial Board: Terms of Reference
The Medical Devices Safety Officers (MDSOs) network is managed by the MDSO Editorial Board and supported by the MHRA and NHS England and NHS Improvement Patient Safety Team. More information on the MDSO network and the relationship between MHRA, NHS England and NHS Improvement can be found in Appendixes 1 and 3. The Editorial Board comprises of volunteers from the MDSO network representing a variety of sectors e.g. NHS acute, community, primary, mental health and independent. The Editorial Board is responsible for agreeing the content of the monthly virtual meeting and setting the strategic direction of the network.

Medical Device Safety Officer Handbook
The purpose of this handbook is to support new as well as established MDSOs to fulfil their role by signposting to relevant information and resources. The Medical Device Safety Officer role was created on 20th March 2014 following the publication of an NHS England Patient Safety Alert that aimed to help healthcare providers increase the quality and frequency of incident reporting for medical device related problems and medication errors. The alert called on large healthcare provider organisations across both public and independent sectors, along with healthcare commissioners, to identify named responsible persons in both medical device and medication safety roles.
MDSO Network Bed Rails FAQ (v1.5 Updated March 2024)
In response to questions received from MDSOs regarding the Bed Rails NatPSA, the MDSO Network has produced this resource in consultation with the MHRA and NHSE and are posting it here for easy access. Please feel free to download and circulate wider with your professional colleagues working on the Bed Rails NatPSA

NHS England & MHRA Stage 3 Directive. Patient Safety Alert 2014: Improving medical device incident reporting and learning NHS/PSA/D/2014/006
The role of the Medical Devices Safety Officer (MDSO)
The establishment of a MDSO role is integral to improving medical device incident reporting and learning within organisations. One of the MDSO’s key roles is to promote the safe use of medical devices across their organisation and provide expert advice. As well as improving the quality of reporting, the MDSO will be the essential link between the identification and implementation of (local and national) medical devices safety initiatives and the daily operations to improve the safety of medical devices.

Supporting Information
The alert recommended changes to strengthen local clinical governance arrangements and the identification of Medical Devices Safety Officers (MDSOs) and multi-professional groups to review incidents and to improve the safety of medical devices. This supporting information provides additional information and clarification on the thinking behind the Patient Safety Alert and recommended actions.
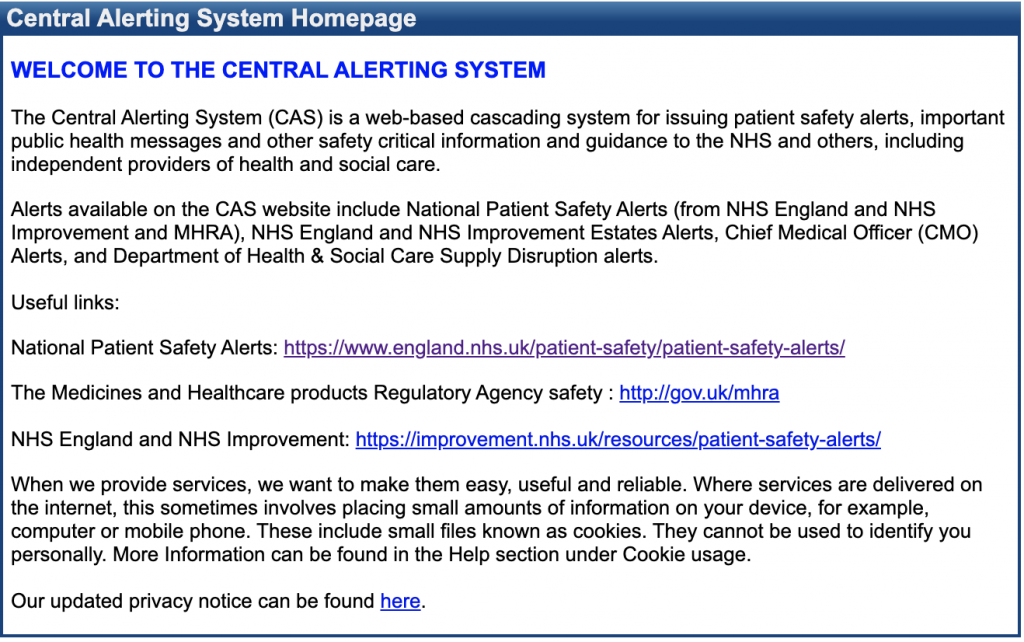
The Central Alerting System
The Central Alerting System (CAS) is a web-based cascading system for issuing patient safety alerts, important public health messages and other safety critical information and guidance to the NHS and others, including independent providers of health and social care. Alerts available on the CAS website include National Patient Safety Alerts (from NHS England and NHS Improvement and MHRA), NHS England and NHS Improvement Estates Alerts, Chief Medical Officer (CMO) Alerts, and Department of Health & Social Care Supply Disruption alerts.

Youtube video on the Role of an MDSO
This video (from 2017) focuses on the establishment of a Medical Device Safety Officer (MDSO) network for the UK and the important part that Clinical Engineering professionals play in this new pivotal role. It is presented by Mr. Paul Lee, Medical Devices Training Manager at Morriston Hospital, Swansea, Wales, UK. This video is a part of UK’s contribution to the Global Clinical Engineering Day on 21 October 2017.